- 1School of Life Sciences, University of Essex, Essex, United Kingdom
- 2Center of Conservation and Biodiversity, University of Exeter, Exeter, United Kingdom
- 3Nekton Foundation, Oxfordshire, United Kingdom
- 4National Oceanography Centre, Southampton, United Kingdom
- 5Ocean Census, Oxfordshire, United Kingdom
The Weddell Sea is one of Earth’s most remote and least studied regions. The region around the Larsen C Ice shelf has been largely inaccessible because of its remoteness, extreme cold, rough seas, ice cover, and deep waters. This study documents the first discovery of maintained nesting sites of Lindbergichthys nudifrons (yellowfin notie) in the western Weddell Sea. Nesting sites were found at all locations surveyed during the Weddell Sea Expedition 2019 onboard the SA Agulhas II using the remotely operated vehicle, Lassie. Unlike previous studies, no significant differences in localised water temperature were detected between nesting sites and surrounding waters, except at one site. Novel nesting patterns, groups of nests close to each other, were discernible throughout the video footage; These patterns are thought to have evolved as a form of group predation protection behaviour. These findings provide critical evidence of unique, structured breeding habitats, fulfilling key criteria for the designation of Vulnerable Marine Ecosystems and strengthening the case for the proposed Weddell Sea Marine Protected Area.
Introduction
Antarctica, the southernmost continent beyond 60° S, is one of Earth’s most remote and extreme environments. Its persistent sea ice, low winter light, and frigid temperatures make it challenging to study. This also provides opportunities to uncover fundamental biological and environmental processes (Vernet et al., 2019; Hutchinson et al., 2020). The Weddell Sea, located within the Southern Ocean, is significant for its biological richness and its contribution to global ocean circulation and climate (Hutchinson et al., 2020). It plays a critical role in forming water mass interactions that drive large-scale ocean currents, regulate global gas exchanges, and influence climate patterns (Foldvik et al., 2004; Vernet et al., 2019). These interactions make the area a hotspot for biological productivity, activity, and abundance (Nachtsheim et al., 2019).
The Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) has proposed the establishment of The Weddell Sea MPA (WSMPA; (Teschke, 2023). This area hosts one of the richest communities in the Antarctic region for fish (Baena et al., 2023), brittle stars (Lau et al., 2021), cephalopods (Staffer et al., 2021; Nesis et al., 1998), sponges (Brey et al., 1994; Brandt et al., 2007; Barthel and Gutt, 1992), marine birds (Reisinger et al., 2022; Teschke et al., 2021), and mammals (Reisinger et al., 2022; Teschke et al., 2020). Breeding grounds or key hunting and foraging areas for cryonotothenioids, marine mammals, and birds all occur within the proposed protection area (Hindell et al., 2020; Fretwell et al., 2012; van Franeker, 1996; Purser et al., 2022).
The suborder Notothenioidei represents a group of fish uniquely adapted to Antarctic environments (Near et al., 2015; La Mesa et al., 2021). They exhibit slow maturation, low fecundity, and large egg production, with most species spawning demersally and showing parental care during incubation, which can exceed 100 days (Everson, 1984; Gon and Heemstra, 1990; La Mesa et al., 2021; Marshall, 1953; Novillo et al., 2022).
The two species in Lindbergichthys have a benthic lifestyle as adults and reach a maximum length of 15cm and 19.5cm for L. mizops and L. nudifrons, respectively (Froese and Pauly, 2022). The latter occupies a greater depth range between 3–400 m compared to 20–220 m for the former. Parental care behaviour has been found in Antarctic icefish, with nesting and egg-guarding being the most common forms (Ferrando et al., 2014; Novillo et al., 2022; Kock et al., 2006). Species in the genus Lindbergichthys often exhibit parental care nesting behaviour, which has been well-studied (Eastman, 2013; La Mesa et al., 2021; Konecki and Targett, 1989). For L. nudifrons, sexual maturity is reached at age 4 to 5 years and length of 8 to 9cm (Hourigan and Radtke, 1989; La Mesa et al., 2017). Females spawn in a nest in late Austral autumn to winter (May to June), usually protected by crevices or rocks (Hourigan and Radtke, 1989). The male guards the nest and eggs, including chasing away egg predators. Post-hatching, larvae migrate to the pelagic zone before returning to the benthos in April (Kellermann, 1989).
Study aims
Considering the challenges of observing the seafloor in Antarctica, many study questions are conducted post-hoc, once exploration has occurred. The Weddell Sea expedition 2019 (WSE) was a multi-disciplinary scientific endeavour to explore habitats around the Larsen C ice shelf that had recently calved. The resulting iceberg, A-68, and its grounding provided a rare opportunity to explore the seabed that had been previously beneath the ice. As part of this voyage of scientific discovery, video of benthic areas was collected. Numerous benthic fish nests were observed. Our aims are to present the resulting analysis of videos taken from this expedition.
Materials and methods
Study site
A major component of the Southern Ocean, the Weddell Sea, is an embayment off the coast of Antarctica between the Antarctic Peninsula in the West and Coats Land in the East. The Weddell Sea Expedition 2019 (WSE) sampling locations were on the North-West Weddell Sea, off the coast of the Antarctic Peninsula (Figure 1).
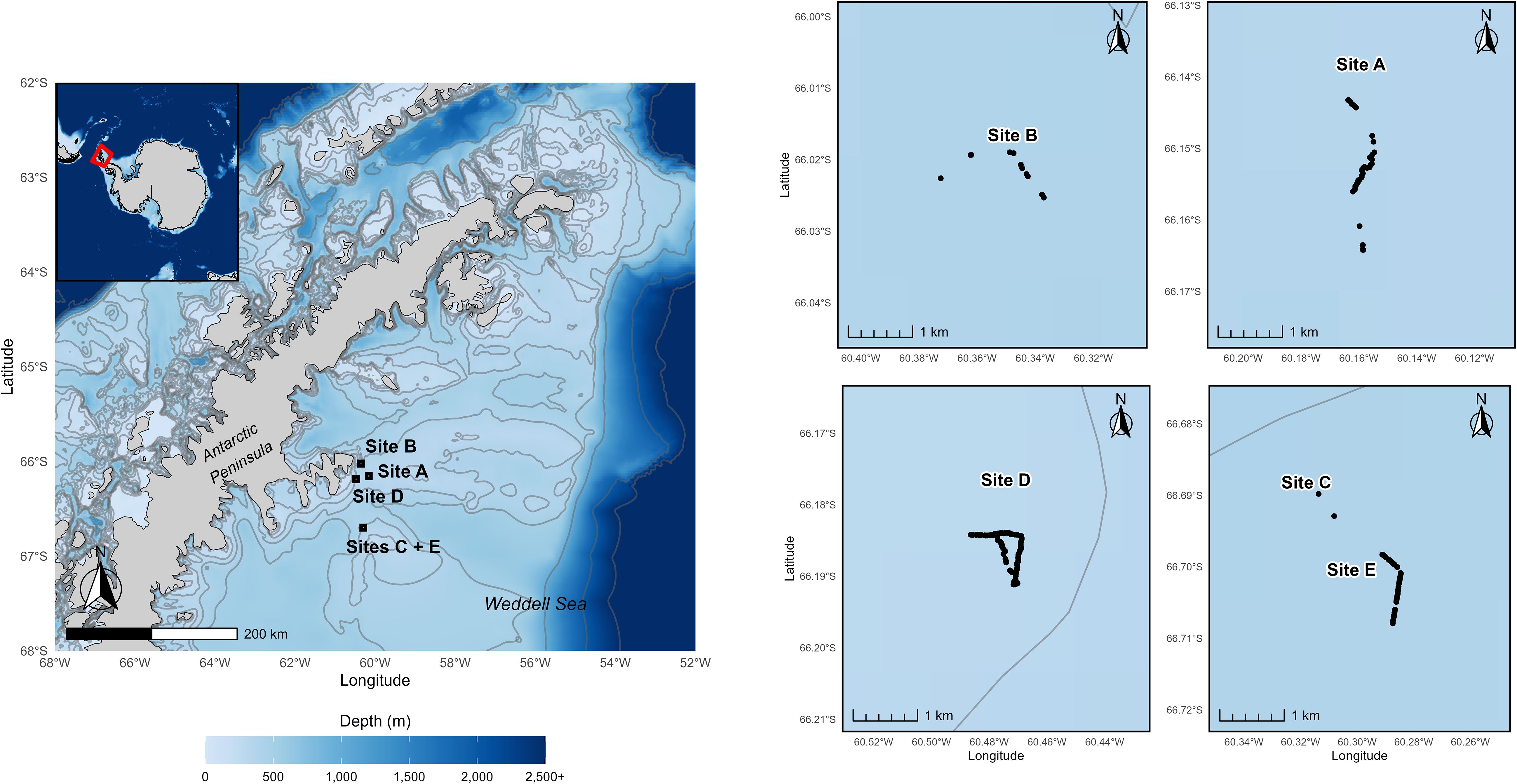
Figure 1. Overview of the nesting site locations. The left panel shows the regional overview of the study area along the East coast of the Antarctic Peninsula. Bathymetry is illustrated with colour shading from 0 to 2,500+ m. Contour intervals are set at 100m intervals to 500m, and then 1000, 2000 and 2500m deep. The inset map provides continental context, with the red rectangle indicating the area shown in the main panel. The black squares on the main map outline the areas detailed in the side panels. The spatial distribution of individual nests (black points) is shown at Site B, Site A, Site D, and Sites C and E. All site maps on the right are presented at the same geographic scale.
Data acquisition and analysis
The WSE was conducted between 1 January and 22 February 2019 (Dowdeswell et al., 2019). Five days (14th January – Site B, 20th January – Site D, 21st January – Site C, 22nd January – Site E, and 23rd January – Site A; Figure 1) were allocated to benthic surveys using the ROV Lassie, with 27 hours of video data collected. The survey locations were selected to represent areas that had been clear of ice cover for varying lengths of time: Site C - 5–10 years, Site D - 15–18 years, and B - 50 years, and Site A, which has been clear of ice cover in glacial history. Seafloor depth ranged between 350–360 m for Site A, 394–407 m for Site B, 392–407 m for Site C, 290–294 m for Site D, and 376–382 m for Site E. The mean maximum depth across all five sites was 376 ± 19m. All video footage captured from the ROV was visually inspected for fish nests, and if found, a snapshot of the video was taken to capture the nests and timestamp. These snapshots were then used to quantify the number of nests, diameter of nests (cm), and unoccupied shells or rock presence (with sizes if present) using ImageJ (Abràmoff et al., 2004). The GPS location and depth were then noted alongside the outputs from the measurement data. All distance measurements were facilitated by the two lasers attached to the ROV at a 10cm distance from each other. Other major epifauna were recorded and identified visually during the video analysis, with emphasis on the surrounding regions of the noted nesting locations. A thermometer measured ambient water temperature around the ROV every 10 seconds, although the Site E data was unretrievable. Due to the lack of sediment samples, the sediment size was estimated visually, and wide characteristics were given for each location. When determining if a nest was present, small depressions in the substratum (those below 6.5cm) were omitted. A large plankton bloom before the video surveys caused flocculent to settle and carpet the seabed, which enabled nests to be classified as ‘inactive’ abandoned nests (flocculent in the depression), and ‘active’ maintained nests (no flocculent inside the depression; Figure 2).

Figure 2. Comparison of non-maintained, abandoned nests (left) and an ‘active’ maintained nest (right) found at Site D. Laser lights have been illustrated with a red line and 10cm annotation.
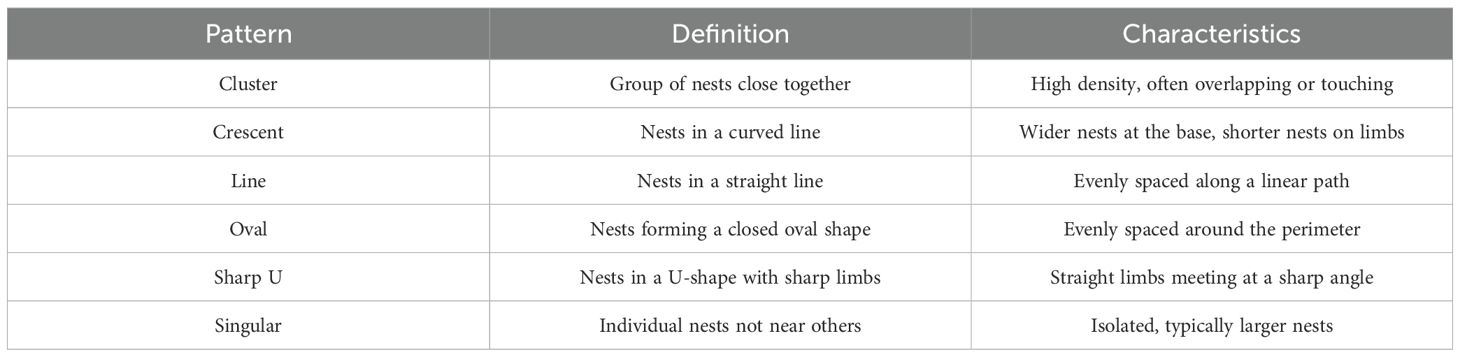
The grouping formations of nests were evaluated and categorised into six nesting patterns (Figure 3), which represented all the configurations seen within the videos. All data analyses and mapping were performed in the open-source software R v.4.2.1 (R Core Team, 2017). The maps were generated using the package ggMap (Kahle and Wickham, 2013). Data collected underwent normality testing, and diameter data had a log transformation to achieve normality. T-test and ANOVA with post-hoc Tukey testing were used to compare the temperature, nesting pattern and site using the ‘stats’ package (R Core Team, 2017). The occurrence of rocks beside nests was also investigated with a generalised linear mixed model with Gaussian distributions to determine whether the rock size has any effect on the size of the nests. Six patterns were present: ‘Cluster’, ‘Crescent’, ‘Line’, ‘Oval’, ‘Sharp U’, and ‘Singular’.

Figure 3. Patterns of cryonotothenioid nests from top left to bottom right; Cluster, Crescent, Line, Oval, Sharp U, and Singular nests.
Results
Nests were generally circular and shallow, parabolic in shape, with the sediment built up on all sides of the nests. Only L. nudifrons were seen in the nests, suggesting these nests were created by this species, which aligns with previous research (Gon and Heemstra, 1990). The average nest size recorded here (12.3cm) was larger than L. nudifrons published size at maturity (9.1 to 9.5cm). The average fish size (10.3cm) was also determined to be greater than their maturation size. Other fishes identified as species other than L. nudifrons were larger (greater than the published maximum length of L. nudifrons) and are known to have larger sizes at maturity (Hourigan and Radtke, 1989). No eggs were seen within any of the nests, presumably as the WSE occurred after known hatching times (Austral Spring; (Hourigan and Radtke, 1989). Some larvae were seen within the nest circumference.
Summary of sample site nesting statistics
A total of 1,036 individual active nests were located across 277 nesting groups on the five Remotely Operated Vehicles (ROV) dives. 93 nests (9%) were classed as inactive and 72 (7%) contained larvae in and/or around the nests. Both active and inactive nests were discovered at depths between 290 and 411 metres. A mean of 2.72 ± 0.22 nests per nesting group was found across all the dives. The most abundant nesting group location was found at site D, with 151 groups of nests, with a mean of 2.3 nests per group. The most abundant location was Site E, with 461 individual nests recorded.
Novel nesting patterns
Six patterns were present: ‘Cluster’, ‘Crescent’, ‘Line’, ‘Oval’, ‘Sharp U’, and ‘Singular’ (Figure 3). The ‘Cluster’ pattern is defined as a group of nests located close to each other without forming a specific shape or structure. The ‘Crescent’ nesting pattern is arranged in a curved line, resembling a crescent moon, and the nests are wider at the base and shorter in diameter on the limbs. The ‘Line’ pattern contains nests that are in an approximate linear path with one other nest, or many other nests. Nests that complete a full ‘Oval’ shape are thus named. Patterns of ‘Sharp U’ consist of a typical ‘U’ shape, where the limbs are perpendicular to each other. The ‘Singular’ pattern is those nests that are not adjacent to nor associated with any other nest.
This is the first report of variable nesting grouping patterns exhibited by cryonotothenioids. The Cluster pattern was represented in 42.08% of all nests, followed by Singular, Sharp U, Oval, Crescent, and Line at 18.82%, 16.8%, 10.14%, 8.5%, and 3.67%, respectively.
There was a significant difference in the diameter of the different nesting patterns (ANOVA; F5,1030 = 13 p < 0.001) (Figure 4). Singular nests had a significantly higher average diameter than those in Cluster, Crescent, Oval, and Sharp U patterns (Tukey; p < 0.001).
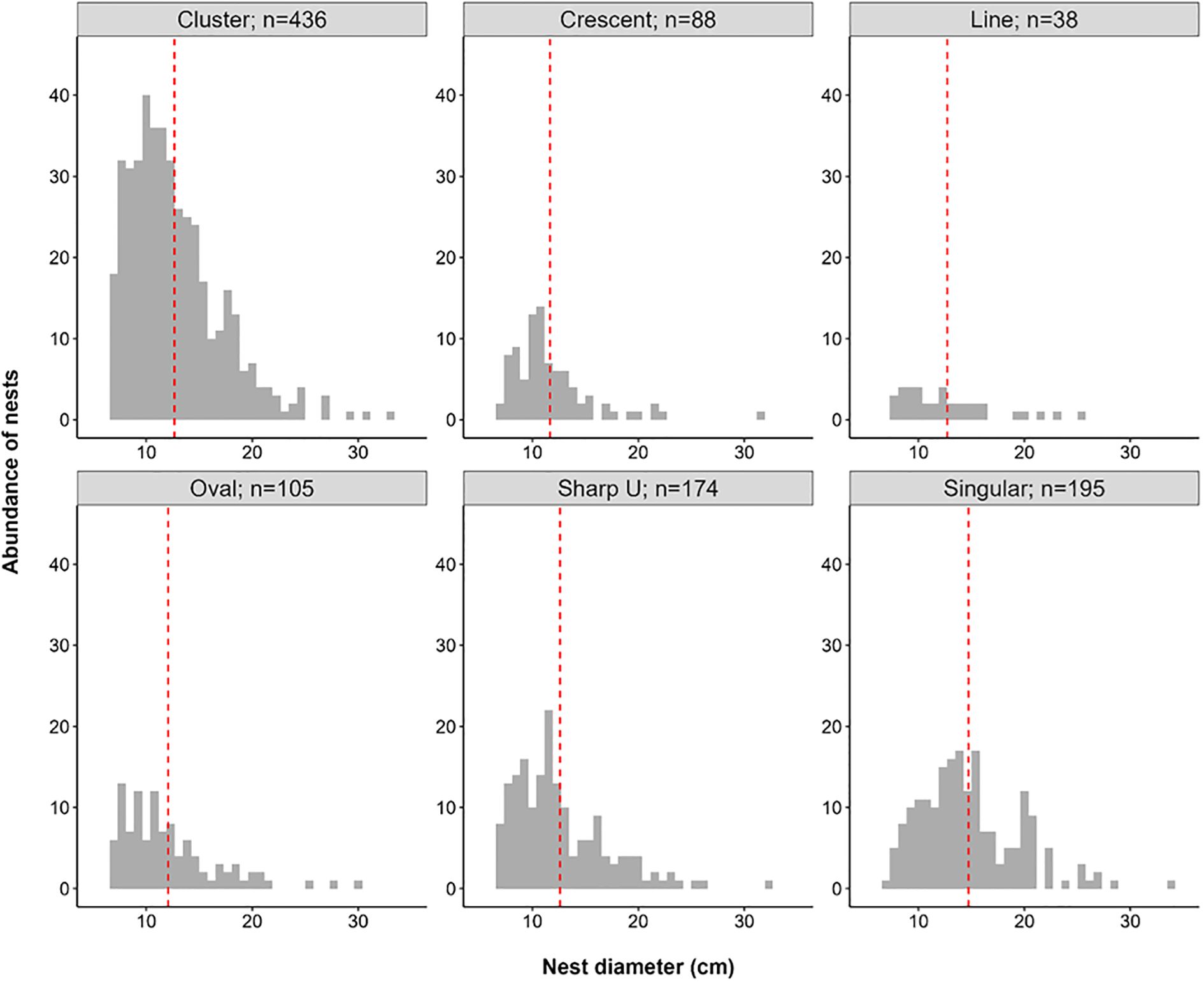
Figure 4. Nesting diameter (cm) abundances faceted by grouping pattern exhibited with the corresponding number of nests found; red dotted line indicates the mean nest diameter (cm).
Nest diameters were significantly different between the sampling sites studied (ANOVA; F4,1031 = 8.44 p < 0.001) (Figure 5). The diameters of the nests found on Site B were significantly lower than Sites D and E (Tukey; p < 0.01, and 0.05, respectively). The nests found in Site A had significantly lower diameters than Sites D and E (Tukey; p < 0.001, and 0.001, respectively).
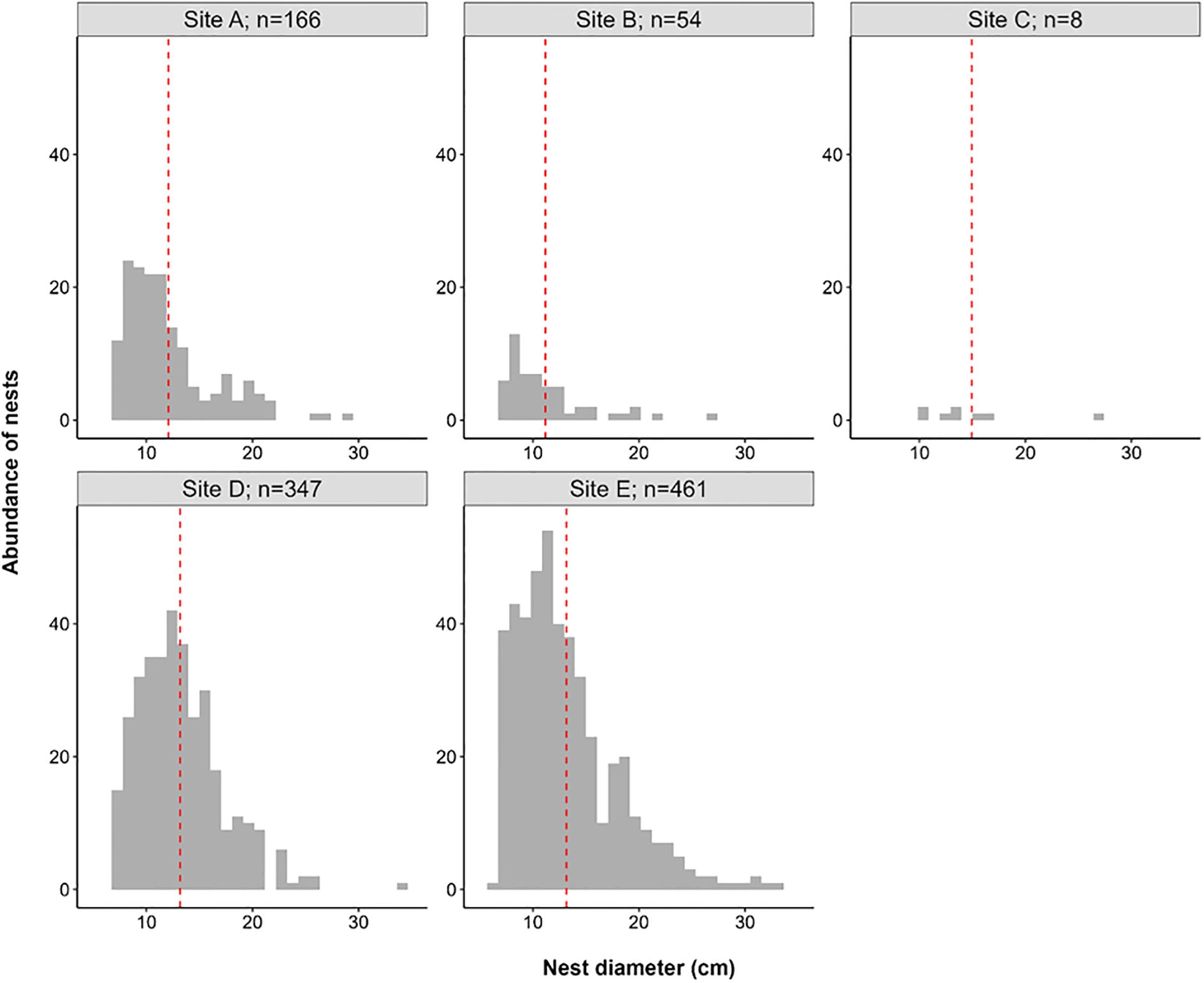
Figure 5. Nesting diameter abundances faceted by site sampled with their corresponding number of nests found, the red dotted line indicates the mean nest diameter (cm).
Distribution of nesting sites
Local temperature distribution ranged between -1.1 °C to -2.09 °C across the nesting sites. The coldest nesting sites were on Site D at 304m deep (-2.04 to -2.09 °C), whereas the warmest site was on Site B at 418m deep (-1.95 to -1.1 °C). The localised nesting temperature in Site C and Site A was -1.7 °C and -1.75 to -1.8 °C, respectively. Localised nesting temperatures did not significantly differ from the surrounding areas (p > 0.05), apart from Site A, where the nesting sites were slightly colder (~0.2 °C) than the surrounding non-nested areas (t-test; t224.39 = -5.1227, p < 0.001).
A total of 154 nests (14.9% of total active nests) had pebbles within and around the nests. Nesting sites adjacent to rocks were seen at every site apart from Site C. Only 213 nests were recorded adjacent to larger rocks, meaning 916 nests were not associated with rocks. Mean rock sizes adjacent to the nests were 26.8 ± 0.86cm. Whilst not significant, the nests adjacent to rocks were smaller, at a mean diameter of 11.85 ± 0.34cm in comparison to 12.42 ± 0.16cm for those not near rocks (p > 0.05). Inside nests, larger rocks at the bottom were seen less frequently (34 nests), but smaller rocks and shells were found aside on the edges of the nests. No relationships were found between the extent of ice-free years and nest characteristics (p > 0.05).
In all locations visited, the visible epifauna were typically low in diversity. Echinoderms and Cnidaria were the most abundant fauna observed, apart from the nests. Among the echinoderms, brittle stars (Ophiuroidea) were the most frequently seen, followed in abundance by feather stars (Crinoidea). Brittle stars were not found inside the nests; however, many were seen on the peak rims or edges of the nests. Individuals were located on the rim in 35% of observed nests. Abundant cnidarians, including Umbellula and Leptogorgia, were observed in the surrounding areas but never physically in contact with nests. Holothurians were also recorded near nests at Sites B, D, E, and A, though these individuals were further away and mainly seen resting. Similarly, the predatory ribbon worm, Parborlasia corrugatus, was seen away from nesting locations. Other less abundant individuals included two morphotypes of siphonophores and an octopus.
Discussion
This study documents a large and widely dispersed nesting aggregation of the yellowfin notothenioid, Lindbergichthys nudifrons, in the western Weddell Sea, an area made accessible by the recent calving of the Larsen C Ice Shelf. The most significant finding is the discovery of six distinct, geometric nesting patterns, which we propose are primarily driven by biotic interactions, namely predation pressure, rather than the abiotic factors observed in other large Antarctic fish breeding colonies. These findings, summarised in Table 1, provide new insights into the complex behavioural ecology of Antarctic notothenioids and underscore the ecological significance of this recently uncovered region.
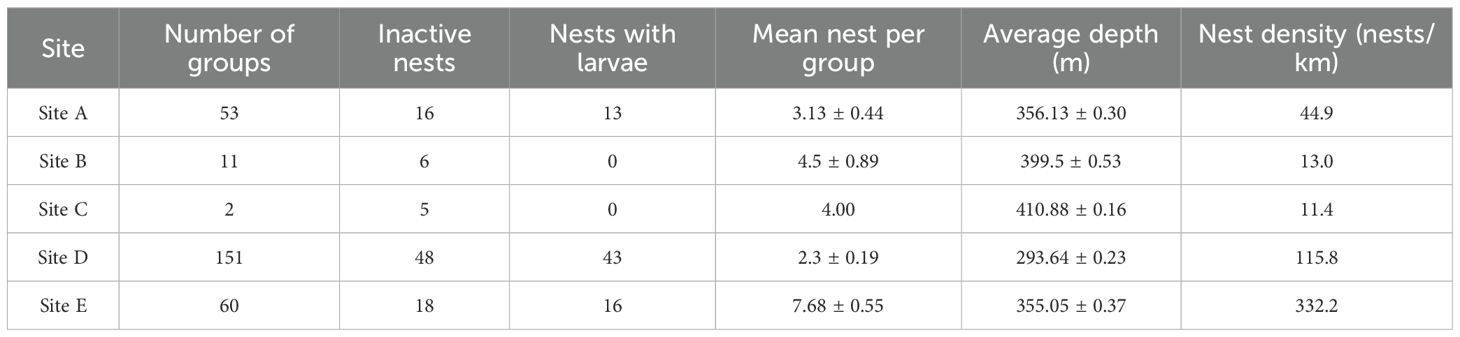
Table 1. Summary of the five sites visited for nesting groups and different nest types (inactive and ones with larvae), mean nests per group (± SE), average site depth (± SE), and the overall nest density calculated per kilometre of the ROV survey track.
The sampling locations were chosen to represent the time since ice coverage, allowing for a comparison between the duration of ice-free conditions and nest characteristics; however, no relationships were found. A more influential factor was the local substratum. Different substrata were found between sites: visually, Site B was rockier, with a high abundance of invertebrate fauna, such as brittle stars, feather stars, and corals. In contrast, the other locations (A, C, D, and E) sediment tended to be finer and hosted more nests and teleosts. This suggests a species preference for softer sediment for nest construction.
This study provides the first documentation of complex, variable nesting patterns for L. nudifrons. The nests themselves were parabolic in structure. The depth of nest depression varied, though this was not possible to measure with precision from the ROV footage. The presence of larger rocks that remained within the nests, alongside smaller pebbles that appeared to have been moved to the nest wall, could be indicative of the amount of energy expended on nest creation. As no fish were observed actively building nests, this remains an area for future investigation. A consistent feature was that in grouped nests, the excavated sediment was typically built up on the sides away from the centre of the overall pattern, suggesting a coordinated construction effort that maintains separation and structure. In a promising sign of reproductive success, fish larvae, identified as L. nudifrons, could be seen floating within the circumference of some nests. The presence of larger rocks beside some nests, particularly evident in the ‘Sharp U’ pattern, is a behaviour that has been noted before in notothenioids (Hourigan and Radtke, 1989). We observed that smaller nests were adjacent to rocks more often than larger nests. These rocks may provide vital shelter from benthic currents or act as enhanced refugia from predation. On a seafloor that is visually rather flat and homogenous, any additional physical complexity, such as a rock, would confer a significant advantage in predation protection, corroborating earlier hypotheses by Hourigan and Radtke (1989).
We identified and categorised six different nesting patterns (Table 2). Of these, ‘Sharp U’, ‘Oval’, and ‘Crescent’ are the most similar in their geometric structure. The observable differences could be attributed to the ‘Sharp U’ and ‘Crescent’ patterns being incomplete or interrupted versions of a full oval. The most common pattern, ‘Cluster’, which accounted for over 42% of nests, lacked a specific geometric shape and was instead a dense accumulation of many nests in a small area. ‘Singular’ nests, the second most abundant pattern, were isolated and had the highest mean nest diameter. This observation could suggest that larger, older and more dominant adults are more comfortable nesting away from the group, being more capable of protecting their nests individually. The ‘Line’ pattern was very distinct and the least abundant. We hypothesise that these patterns are an anti-predator adaptation. The rarity of the ‘Line’ pattern, for example, could be associated with a reduction in its effectiveness for community anti-predator adaptation, as no single nest achieves a centrally protected position. In contrast, the ‘Cluster’ pattern strongly aligns with the selfish herd theory proposed by Hamilton (1971), where individuals reduce their domain of danger by putting other individuals between themselves and an approaching predator. This would offer a significantly reduced risk to the nests in the centre of the cluster. Such defensive patterning, while novel for this species, is a well-documented phenomenon in fish nesting colonies in shallow tropical reef scenarios, where it is also attributed to increased predation protection (Gross and MacMillan, 1981; Tyler Iii, 1995). Previous research on L. nudifrons has shown that guarding males will defend a territory up to 25cm away from their nest (Hourigan and Radtke, 1989). In the dense patterns recorded here, this defence zone would frequently encroach on their neighbour’s nests, suggesting a high level of tolerance and a communal defensive posture.
This defensive behaviour is likely a direct response to the local predators. The epifauna observed near the nests provides several candidates. It is possible that scavenging brittle stars (Ophiuroidea) positioned on the nest edges were predating on cryonotothenioid eggs, a behaviour previously noted from stomach content analysis (Volage et al., 2021; Fratt and Dearborn, 1984). The strategic location of these brittle stars also suggests they may be taking advantage of food sources carried by water currents that are channelled over the nests. A more significant threat, however, may be the predatory nemertean, Parborlasia corrugatus. This species is a voracious scavenger and predator and was observed in the general area (Gibson, 1983). We propose that the complex nesting patterns may serve to reduce predation by P. corrugatus. As this predator utilises chemotactic mechanisms to actively search for food, the aggregated nests may disguise or dilute the individual odour plumes from the eggs. This could create a confusing sensory environment for the nemertean, making it more difficult to detect and target a single nest, a crucial adaptive strategy during the long incubation period. Other fauna were present but appeared to have neutral interactions; the absence of physical contact between cnidarians like Umbellula sp. and Leptogorgia sp. and the nests could imply a neutral relationship, while the resting behaviour of holothurians near the nests may indicate a low-energy interaction with the environment.
Two key unresolved questions remain regarding the creators of these nests: the social structure and the certainty of the species identification. It is not known whether one mating pair created all the nests in a group or whether each nest was attributed to a different, single mating pair; however, it is presumed that each nest was for a single pair, given the high energetic costs and significant predation risk involved in guarding even one nest. Furthermore, a necessary limitation of this study is the certainty of species attribution for every observed nest. Our attribution to L. nudifrons is based on strong visual evidence; across 27 hours of video, it was the only species directly observed occupying or actively maintaining the nests. However, in the absence of a guarding fish or eggs at every nest, we cannot definitively exclude the possibility that a minority were created by other cryonotothenioid species. This is a particularly relevant caveat, as a recent study have shown species determination in this group is problematic (Schiavon et al., 2023), highlighting a challenge common to deep-sea visual surveys.
A striking finding of this study is the lack of a strong, localised abiotic driver for nest location, particularly when compared to other known Antarctic fish colonies. Except for Site A, our data show no significant difference between the water temperature at nesting locations and the surrounding, nest-free waters. Site B, the warmest site, exhibited slightly smaller nest diameters. Although the temperature difference across all sites is marginal (approximately 0.2 °C), it is worth considering its biological relevance. Studies on ectothermic Antarctic fish suggest that even such minor temperature variations can influence metabolic rates (Clarke, 1983; Sandersfeld et al., 2017; Enzor and Place, 2014; Johnston et al., 1991). However, the considerable overlap in temperature ranges between the sites, coupled with the lack of a consistent trend, suggests this small difference is not sufficient to induce significant biological effects such as changes in nest size or recruitment. While it is uncertain whether this temperature change affects developmental time, it requires further investigation.
This stands in stark contrast to the large Neopagetopsis ionah breeding colony reported by Purser et al. (2022) (Table 3). That site was defined by a dominant inflow of modified warm deep water (mWDW) that was up to 2 °C warmer than the surrounding bottom water, a feature hypothesised to be the key factor driving nest site selection. While it is tempting to draw parallels, the short-term nature of both temperature data, alongside the fact that the temperature change in the N. ionah colony was an order of magnitude greater, limits our ability to make a direct comparison and underscores a fundamental difference between the two sites. Since the waters of the high Antarctic shelf are known for their thermal stability, even small temperature variations may be ecologically significant, but without long-term monitoring, it is difficult to assert whether these differences represent a consistent environmental factor.
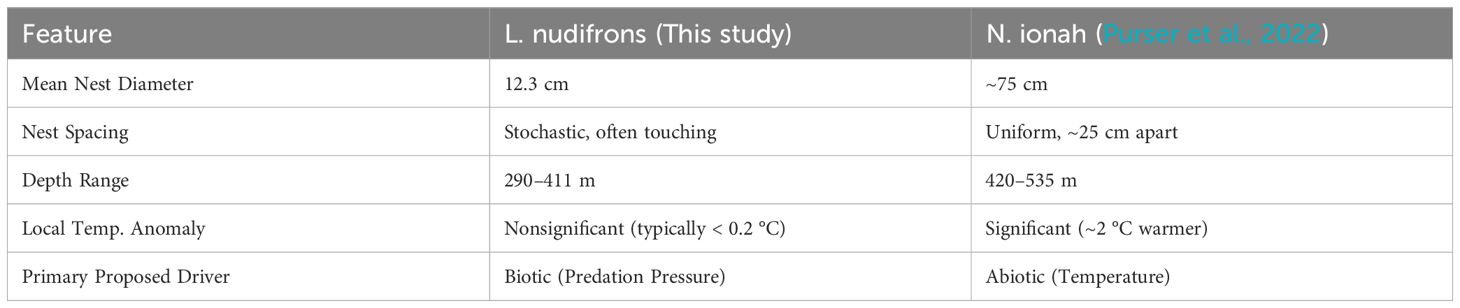
Table 3. The differing drivers and characteristics of these two major Weddell Sea nesting sites (this study, and Purser et al. (2022)).
The broader oceanography of our study region is certainly a contributing factor to its overall suitability. The mWDW has been shown to reach this location, mixing with colder, denser Ice Shelf Water (ISW) and High Salinity Shelf Water (HSSW). This mixing of local shelf water masses and the mWDW is known to drive a greater level of local productivity, likely creating a favourable environment for a greater abundance of nesting sites across this area. However, our data suggest this is a regional, rather than a localised, driver. These oceanographic processes and how they influence the selection of broad nesting areas, versus the fine-scale patterns within them, are an advised focus of future research.
The physical characteristics of the nests also differed logically with species size, as did the water depths, which conformed with each species’ published depth ranges and habitat preferences. Spacing between L. nudifrons nesting sites was more stochastic, and neighbouring nests were often touching, whereas at the N. ionah site, nests were spaced ~25 cm away from each other. Furthermore, the timing of the two expeditions revealed different stages of the reproductive cycle, suggesting the wider Weddell Sea is a critical site of species-dependent nesting for over half the year.
Correctly identifying and defining these unique biological features is crucial for their conservation. A recent proposal by Teschke (2023) suggested the terms ‘nest’ and ‘potential nest’, which are established by the presence of eggs for the former, and the absence of eggs but with one or more fish present for the latter. Due to our expedition’s timing post-hatching, it was not possible to confirm the presence of eggs. However, we were able to confidently identify occupied, active nesting sites because phytodetritus was consistently ‘cleaned’ from the nests by adult fish as a form of maintenance behaviour, whereas the surrounding, unmaintained seafloor was carpeted in this detritus. This method provides a reliable proxy for identifying active nesting sites outside of the spawning season.
This discovery has immediate and significant conservation importance. Within the 43rd meeting of the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR), the Antarctic and Southern Ocean Coalition agreed that clear video evidence of fish nesting sites is required to create a Conservation Measure. Our results provide precisely this evidence for one of the few documented fish nesting sites in the entire Weddell Sea. Spawning and nesting sites play a crucial role in the wider spatial conservation landscape. They are key features used to identify Vulnerable Marine Ecosystems (VMEs) under the FAO Guidelines for Management of Deep-sea Bottom Fisheries in the High Seas and are also identified as features of Ecologically and Biologically Significant Areas (EBSAs) under the Convention on Biological Diversity (CBD). Our findings match the criteria and designations for these respective conservation measures. This work, therefore, underscores the critical importance of protecting these unique habitats and provides robust evidence for the designation of the Weddell Sea Marine Protected Area (MPA).
Conclusion
In conclusion, this study presents the discovery of a large, active, and well-dispersed cryonotothenioid nesting habitat in the Western Weddell Sea. The differing nesting patterns are the first described for this species and are strongly speculated to be a group behaviour for predation evasion. Crucially, the nesting sites were not shown to have any site-specificity driven by the tested abiotic variables, such as localised temperature. This suggests that biotic interactions are the primary drivers of this complex aggregation, a significant contrast to other known notothenioid colonies. This research also provides valuable ground-truthing for the cryonotothenioid nesting site suitability model from Teschke et al. (2016) and furthers the discussion into nesting site definitions as proposed by Teschke (2023). The extensive, active nesting sites documented here provide further compelling evidence to support the designation of the proposed Weddell Sea Marine Protected Area.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Essex Ethical Approval Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. AR: Conceptualization, Funding acquisition, Resources, Validation, Writing – review & editing. MT: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The research leading to these results received funding from the Flotilla Foundation. The authors have no relevant financial or non-financial interests to disclose.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abràmoff M. D., Magalhães P. J., and Ram S. J. (2004). Image processing with imageJ. Biophotonics Int. 11, 36–42.
Baena P., Santín A., La Mesa M., Riginella E., Owsianowski N., Gili J.-M., et al. (2023). Are there distribution patterns and population structure differences among demersal fish species in relation to Antarctic benthic communities? A case study in the Weddell Sea. Polar Biol. 46, 1069–1082. doi: 10.1007/s00300-023-03184-y
Barthel D. and Gutt J. (1992). Sponge associations in the eastern Weddell Sea. Antarctic Sci. 4, 137–150. doi: 10.1017/S0954102092000221
Brandt A., Gooday A. J., Brandão S. N., Brix S., Brökeland W., Cedhagen T., et al. (2007). First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 447, 307–311. doi: 10.1038/nature05827
Brey T., Klages M., Dahm C., Gorny M., Gutt J., Hain S., et al. (1994). Antarctic benthic diversity. Nature 368, 297–297. doi: 10.1038/368297a0
Clarke A. (1983). Life in cold water: the physiological ecology of polar marine ectotherms. Oceanography Mar. Biol. 21, 341–453.
Dowdeswell J., Shears J., Batchelor C., Christie F., Rack W., Montelli A., et al. (2019). The Weddell Sea Expedition 2019: Cruise Scientific Report. Apollo - University of Cambridge Repository. doi: 10.17863/CAM.58103
Eastman J. T. (2013). Antarctic Fish Biology: Evolution in a Unique Environment (San Diego, California, USA: Academic Press).
Enzor L. A. and Place S. P. (2014). Is warmer better? Decreased oxidative damage in notothenioid fish after long-term acclimation to multiple stressors. J. Exp. Biol. 217, 3301–3310. doi: 10.1242/jeb.108431
Everson I. (1984). Fish biology. In: Laws R. M. (ed), Antarctic ecology, Vol. 2, Academic Press, London, pp 491–532.
Ferrando S., Castellano L., Gallus L., Ghigliotti L., Masini M. A., Pisano E., et al. (2014). A demonstration of nesting in two Antarctic icefish (Genus chionodraco) using a fin dimorphism analysis and ex situ videos. PloS One 9, e90512. doi: 10.1371/journal.pone.0090512
Foldvik A., Gammelsrød T., Østerhus S., Fahrbach E., Rohardt G., Schröder M., et al. (2004). Ice shelf water overflow and bottom water formation in the southern Weddell Sea. J. Geophysical Research: Oceans 109, C02015. doi: 10.1029/2003JC002008
Fratt D. B. and Dearborn J. H. (1984). Feeding biology of the Antarctic brittle star Ophionotus victoriae (Echinodermata: Ophiuroidea). Polar Biol. 3, 127–139. doi: 10.1007/BF00442644
Fretwell P. T., LaRue M. A., Morin P., Kooyman G. L., Wienecke B., Ratcliffe N., et al. (2012). An emperor penguin population estimate: the first global, synoptic survey of a species from space. PloS One 7, e33751. doi: 10.1371/annotation/32c246eb-3b73-4410-a44c-b41ddae11fc5
Froese R. and Pauly D. (2022). Fishbase. Version 02/2022 Vol. 3 (World Wide Web Electronic Publication). Available online at: https://fishbase.org/ (Accessed July 14, 2024).
Gibson R. (1983). Antarctic nemerteans: the anatomy, distribution and biology of Parborlasia corrugatus (McIntosh 1876)(Heteronemertea, Lineidae). Biol. Antarctic Seas XIV 39, 289–316. doi: 10.1029/AR039p0289
Gon O. and Heemstra P. C. (1990). Fishes of the Southern Ocean (Grahamstown: JLB Smith Institute of Ichthyology Grahamstown).
Gross M. R. and MacMillan A. M. (1981). Predation and the evolution of colonial nesting in bluegill sunfish (Lepomis macrochirus). Behav. Ecol. Sociobiology 8, 163–174. doi: 10.1007/BF00299826
Hamilton W.D. (1971). Geometry for the selfish herd. J. Theor. Biol. 31 (2), 295–311. doi: 10.1016/0022-5193(71)90189-5
Hindell M. A., Reisinger R. R., Ropert-Coudert Y., Hückstädt L. A., Trathan P. N., Bornemann H., et al. (2020). Tracking of marine predators to protect Southern Ocean ecosystems. Nature 580, 87–92. doi: 10.1038/s41586-020-2126-y
Hourigan T. F. and Radtke R. L. (1989). Reproduction of the Antarctic fish Nototheniops nudifrons. Mar. Biol. 100, 277–283. doi: 10.1007/BF00391969
Hutchinson K., Deshayes J., Sallee J.-B., Dowdeswell J. A., de Lavergne C., Ansorge I., et al. (2020). Water mass characteristics and distribution adjacent to larsen C ice shelf, Antarctica. J. Geophysical Research: Oceans 125, e2019JC015855. doi: 10.1029/2019JC015855
Johnston I. A., Clarke A., and Ward P. (1991). Temperature and metabolic rate in sedentary fish from the Antarctic, North Sea and Indo-West Pacific Ocean. Mar. Biol. 109, 191–195. doi: 10.1007/BF01319386
Kahle D. J. and Wickham H. (2013). ggmap: spatial visualization with ggplot2. R J. 5, 144. doi: 10.32614/RJ-2013-014
Kellermann A. K. (1989). Catalogue of early life history stages of Antarctic Nototheniidae fish. In: Kellermann A. (ed) Identification key and catalogue of larval antarctic fishes. BIOMASS Sci Ser 10, 45–136.
Kock K. H., Pshenichnov L. K., and Devries A. L. (2006). Evidence for egg brooding and parental care in icefish and other notothenioids in the Southern Ocean. Antarctic Sci. 18, 223–227. doi: 10.1017/S0954102006000265
Konecki J. T. and Targett T. E. (1989). Eggs and larvae of Nototheniops larseni from the Spongocoel of a Hexactinellid sponge near Hugo island, Antarctic Peninsula. Polar Biol. 10, 197–198. doi: 10.1007/BF00238495
La Mesa M., Llompart F., Riginella E., and Eastman J. T. (2021). Parental care and reproductive strategies in notothenioid fishes. Fish Fisheries 22, 356–376. doi: 10.1111/faf.12523
La Mesa M., Riginella E., Catalano B., Jones C. D., and Mazzoldi C. (2017). Maternal contribution to spawning and early life-history strategies of the genus Lepidonotothen (Nototheniidae, Perciformes) along the southern Scotia Arc. Polar Biol. 40, 1441–1450. doi: 10.1007/s00300-016-2068-x
Lau S. C. Y., Strugnell J. M., Sands C. J., Silva C. N. S., and Wilson N. G. (2021). Evolutionary innovations in Antarctic brittle stars linked to glacial refugia. Ecol. Evol. 11, 17428–17446. doi: 10.1002/ece3.8376
Marshall N. B. (1953). Egg size in arctic, Antarctic and deep-sea fishes. Evolution 7, 328–341. doi: 10.2307/2405343
Nachtsheim D. A., Ryan S., Schröder M., Jensen L., Oosthuizen W. C., Bester M. N., et al. (2019). Foraging behaviour of Weddell seals (Leptonychotes weddellii) in connection to oceanographic conditions in the southern Weddell Sea. Prog. Oceanography 173, 165–179. doi: 10.1016/j.pocean.2019.02.013
Near T. J., Dornburg A., Harrington R. C., Oliveira C., Pietsch T. W., Thacker C. E., et al. (2015). Identification of the notothenioid sister lineage illuminates the biogeographic history of an Antarctic adaptive radiation. BMC evolutionary Biol. 15, 1–14. doi: 10.1186/s12862-015-0362-9
Nesis K. N., Nigmatullin C. M., and Nikitina I. V. (1998). Spent females of deepwater squid Galiteuthis glacialis under the ice at the surface of the Weddell Sea (Antarctic). J. Zoology 244, 185–200. doi: 10.1111/j.1469-7998.1998.tb00024.x
Novillo M., Desvignes T., Moreira E., and Barrera-Oro E. (2022). Egg predation in Antarctic fish: the ingestion by Notothenia coriiceps of an entire Trematomus bernacchii spawn identified by molecular techniques. Estuarine Coast. Shelf Sci. 266, 107742. doi: 10.1016/j.ecss.2022.107742
Purser A., Hehemann L., Boehringer L., Tippenhauer S., Wege M., Bornemann H., et al. (2022). A vast icefish breeding colony discovered in the Antarctic. Curr. Biol. 32, 842–850. doi: 10.1016/j.cub.2021.12.022
R Core Team (2017). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). https://www.R-project.org/.
Reisinger R. R., Brooks C. M., Raymond B., Freer J. J., Cotté C., Xavier J. C., et al. (2022). Predator-derived bioregions in the Southern Ocean: Characteristics, drivers and representation in marine protected areas. Biol. Conserv. 272, 109630. doi: 10.1016/j.biocon.2022.109630
Sandersfeld T., Mark F. C., and Knust R. (2017). Temperature-dependent metabolism in Antarctic fish: do habitat temperature conditions affect thermal tolerance ranges? Polar Biol. 40, 141–149. doi: 10.1007/s00300-016-1934-x
Schiavon L., Negrisolo E., Battistotti A., Lucassen M., Damerau M., Harms L., et al. (2023). Species identification and population genetics of the Antarctic fish genera Lepidonotothen and Nototheniops (Perciformes, Notothenioidei). Zoologica Scripta 52, 136–153. doi: 10.1111/zsc.12580
Staffer J., Schwarz R., Purser A., Hehemann L., and Hoving H.-J. T. (2021). “Deep seafloor imagery from the Weddell Sea document natural food falls and octopod diversity and distribution,” in EPIC316th Deep Sea Biology Symposium, Brest, France, September 2021 - September 2021.
Teschke K., Pehlke H., Deininger M., Jerosch K., and Brey T. (2016): Scientific background document in support of the development of a CCAMLR MPA in the Weddell Sea (Antarctica) – Version 2016 - Part C: Data analysis and MPA scenario development. Other
Teschke K. (2023). Fish nest area in the southern Weddell Sea: Discussions and recommendations of CCAMLR-41 and a proposal for further action.
Teschke K., Brtnik P., Hain S., Herata H., Liebschner A., Pehlke H., et al. (2021). Planning marine protected areas under the CCAMLR regime–The case of the Weddell Sea (Antarctica). Mar. Policy 124, 104370. doi: 10.1016/j.marpol.2020.104370
Teschke K., Pehlke H., Siegel V., Bornemann H., Knust R., and Brey T. (2020). An integrated compilation of data sources for the development of a marine protected area in the Weddell Sea. Earth Syst. Sci. Data 12, 1003–1023. doi: 10.5194/essd-12-1003-2020
Tyler Iii W. A. (1995). The adaptive significance of colonial nesting in a coral-reef fish. Anim. Behav. 49, 949–966. doi: 10.1006/anbe.1995.0125
van Franeker J. A. (1996). Pelagic distribution and numbers of the Antarctic petrelThalassoica Antarctica in the Weddell Sea during spring. Polar Biol. 16, 565–572. doi: 10.1007/BF02329053
Vernet M., Geibert W., Hoppema M., Brown P. J., Haas C., Hellmer H. H., et al. (2019). The Weddell gyre, southern ocean: present knowledge and future challenges. Rev. Geophysics 57, 623–708. doi: 10.1029/2018RG000604
Keywords: Antartica, cryonotothenioid, Lindbergichthys nudifrons, nesting patterns, Western Weddell Sea
Citation: Connelly RB, Woodall LC, Rogers AD and Taylor ML (2025) A finding of maintained cryonotothenioid nesting sites in the Western Weddell Sea. Front. Mar. Sci. 12:1648168. doi: 10.3389/fmars.2025.1648168
Received: 16 June 2025; Accepted: 29 August 2025;
Published: 29 October 2025.
Edited by:
Ricardo Serrão Santos, University of the Azores, PortugalReviewed by:
Autun Purser, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), GermanyJoão Pedro Barreiros, University of the Azores, Portugal
Copyright © 2025 Connelly, Woodall, Rogers and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Russell B. Connelly, ci5jb25uZWxseUBlc3NleC5hYy51aw==
†ORCID: Russell B. Connelly, orcid.org/0009-0001-5245-0252
Lucy C. Woodall, orcid.org/0000-0001-7295-7184
Alex David Rogers, orcid.org/0000-0002-4864-2980
Michelle L. Taylor, orcid.org/0000-0001-7271-4385
 Russell B. Connelly
Russell B. Connelly Lucy C. Woodall
Lucy C. Woodall Alex David Rogers
Alex David Rogers Michelle L. Taylor
Michelle L. Taylor